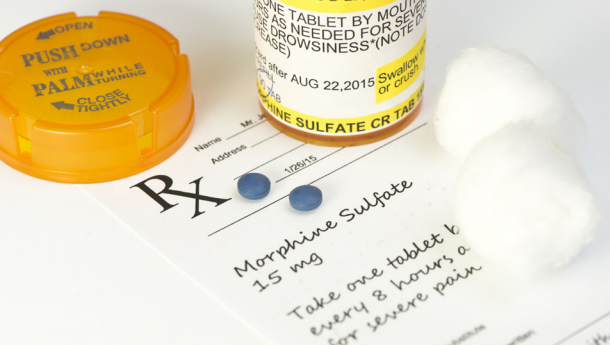
If you prescribe opioids in your practice, it is important to be familiar with current clinical practice guidelines for prescribing opioids, DEA requirements, and federal and state laws and regulations to reduce your professional and administrative liability risk. In this article we will discuss some of the guidelines, requirements, and regulations relating to prescribing opioids.
Prescribing Opioids – Important Information for Practitioners
Apr 17, 2024 4:54:43 PM / by PICA Risk Management Specialist posted in Risk Management, Compliance
Managing Patient Expectations Post-Surgery
Mar 12, 2024 4:08:15 PM / by PICA Risk Management Specialist posted in Risk Management
Over the years, there have been many lawsuits filed against physicians because the patient was not satisfied with a surgical outcome. It could have been a post-operative complication, delayed healing, prolonged pain, cosmetically undesirable result, or a host of other reasons. In the majority of these cases, the physician was found to have acted within the standard of care and the case was eventually dropped or in the event the case went to trial, the jury found in favor of the physician. In the meantime, a lot of time, effort, money, frustration, and stress was spent on claims in which the medicine was good.
So, Why Do These Patients Sue?
This quote by S. Jay Jayasankar, MD is insightful, “The patient’s expectation, not ours, is the yardstick by which our patients measure the course of recovery, occurrence of complications, and the outcome.” If the patient’s expectations are not met, the patient is more apt to sue, regardless of whether malpractice occurred.
Managing Patient Expectations Pre-Operatively
Feb 16, 2024 10:49:06 AM / by PICA Risk Management Specialist posted in Risk Management
Regardless of how long you've been practicing, you have undoubtedly come across a patient that has unrealistic expectations for surgery. This typically manifests in an unhappy post-operative patient. The patient may complain that their scar is too big, their recovery is taking too long, they are not able to return to work when they want, they cannot return to sports activities as quickly as they would like, they can’t wear stilettos, and the list goes on. And as we all know, an unhappy patient is more likely to file a lawsuit than a happy patient.
Complications Resulting in Amputation – Reducing the Risk for You and Your Patients
Jan 11, 2024 2:14:40 PM / by PICA Risk Management Specialist posted in Risk Management
As you could probably surmise, amputations are a leading factor in malpractice suits against podiatrists. So, how do you reduce the risk that a complication will result in amputation? And if amputation is necessary, how can you reduce the risk of a lawsuit being filed?
Communicating Unexpected Outcomes to Patients
Jan 11, 2024 2:02:36 PM / by PICA Risk Management Specialist posted in Risk Management
As a practitioner, you have most likely experienced an unexpected outcome in your care and treatment of a patient. It does not mean you are a bad physician. It happens even with the most experienced physician, but it can be very distressing for you and your patient. How can you protect yourself from risk and maintain a healthy physician-patient relationship in the event of an unexpected outcome?
Start at the Beginning
First, prepare your patients for the possibility of an unexpected outcome. At the beginning when you’re developing a treatment or surgical plan with your patient, discuss the risks and benefits of your plan, including the most likely complications or side effects. Include a discussion of any risk factors that the patient may have that might affect the success of your plan. For example, a patient would be more likely to experience post-operative complications if they are a smoker. With medically complex patients, discuss the treatment challenges and the fact that they may require treatment from a team of specialists/healthcare professionals.
More on Amniotic Fluid Injections
Jul 19, 2023 9:57:00 AM / by J. Kevin West posted in Risk Management, Compliance
We continue to receive questions from podiatry practices regarding the use of and billing for amniotic fluid injections for musculoskeletal purposes. We recently published an article, “Caveat Emptor Vendor: Skin Substitutes & Injectable Amniotic Fluid” that details some real-life examples of the risk providers incur if they fail to do proper due diligence in these situations. In the article below, we continue the conversation around amniotic fluid injections by answering three of the most asked questions so you can make informed decisions at your practice.
Caveat Emptor Vendor: Skin Substitutes & Injectable Amniotic Fluid
Jun 13, 2023 1:52:15 PM / by J. Kevin West posted in Risk Management, Compliance
In the past year, we have seen a dramatic uptick in audits and overpayment claw backs involving two high-dollar products: skin substitutes for wound care and injectable amniotic fluid used for musculoskeletal conditions. While there is no question that these products work, practitioners are often guilty of listening uncritically to sales pitches by vendors who promise high reimbursement and certain payer coverage. Failure to conduct due diligence on these products puts providers at high financial risk because of the substantial cash outlay required to purchase the products, typically upfront.
DVT/PE Prophylaxis for Podiatric Surgery and Treatment Requiring Immobilization – Is it Necessary?
Jun 7, 2023 3:56:55 PM / by PICA Risk Management Specialist posted in Risk Management
It is not uncommon for a podiatric physician to be named in a lawsuit when a patient suffers a DVT/PE following podiatric surgery or treatment requiring immobilization. Allegations against the physician commonly include:
Make Your Documentation More Meaningful for You and Your Patients
May 18, 2023 12:26:55 PM / by PICA Risk Management Specialist posted in Risk Management
All physicians know a medical record must be maintained on each patient. Why? Medical record documentation is required by state and federal laws. Proper documentation is important for continuity of patient care and necessary to receive reimbursement for services rendered. The patient’s medical record is a legal record of the care you provide to the patient and is a valuable tool in your defense should a medical malpractice lawsuit be brought against you.
Retirement Planning: Are You Prepared?
Apr 13, 2023 10:55:38 AM / by PICA Risk Management Specialist posted in Risk Management, Practice Management
If you’re thinking about retirement, it’s prudent to start planning well in advance. If you are a solo practitioner, will you sell or wind down your practice? If you are in a group practice or employed, what are your obligations regarding providing notice of retirement? What are your state licensing board’s requirements regarding retirement? How will you retain your medical and business records? How will you notify your staff and patients of your plan to retire? What do your contracts with health insurers require when you retire from practice? Do you need to purchase professional liability insurance “tail coverage”?